Tyrosine kinase inhibitor (TKI) therapies have profoundly changed the natural history and survival of CML patients. However, the majority of the patients remain on long-lasting therapies and switch one TKI to another, either because of intolerance or resistance. All TKI cessation trials showed approximately 50% of leukemia recurrence rates even after several years of TKI therapies, suggesting the persistence of residual leukemic stem cells in patients in deep molecular response (DMR). The resistance and persistence of CML HSC are related to intrinsic (BCR-ABL independent survival) or extrinsic (leukemic niche ) mechanisms some of which have been extensively studied. However, there has been no data with regard to the role of asymmetric cell division pathways in the persistence and progression of CML stem cell clone. Indeed, During asymmetric division process of hematopoietic stem cells, some proteins are differently segregated between daughter HSCs such as endosome associated tetraspanin proteins (CD53, CD63) and mitochondria-associated fission regulator Dynamin related protein-1(DRP1). Similarly, it has been shown that asymmetric organelle inheritance can influence HSC decisions towards differentiation versus self-renewal. Interestingly, the tetraspanin CD63 is known to be involved in the quiescence of human and murine HSC via activation of TGF-beta pathway, CD63 hi cells exhibiting increased self-renewal potential after the asymmetric division with reduced differentiation potential.
Materials and Methods
We have analyzed the transcriptome performed in a cohort of CML patients in chronic phase (CP) and blast phase (BP) (GSE4170) as well as in a validation cohort of RNA-sequencing (GSE100026). Similarly, single cell transcriptome analysis of Lin- CD34+ CD38- CML progenitors (GSE76312) were included in the study evaluating the expression of genes involved in asymmetric cell division. We have then generated an asymmetric hematopoietic stem cell risk score using a panel of genes involved in the asymmetric division of HSCs and correlated these results with the transcriptome of the CML cohorts above. A pseudotime analysis was performed to evaluate single cell trajectories of the genes identified.
Results
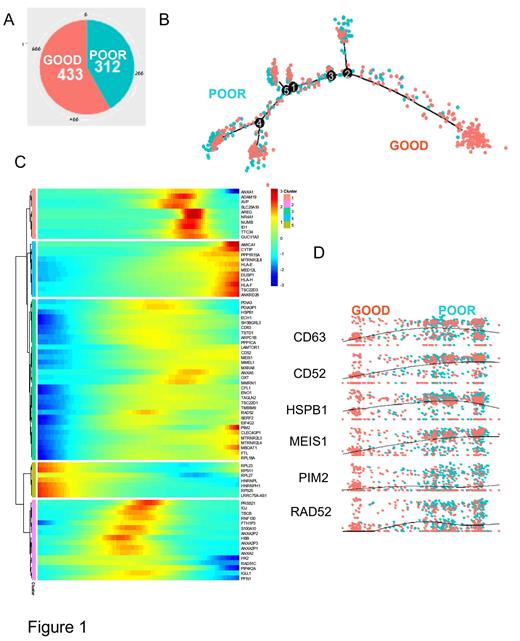
To determine the potential role of asymmetric cell division markers in the progression of CML, we have analyzed the expression of several of these genes during CML progression in two independent transcriptome cohorts. The regulation concordance between the two distinct cohorts was principally observed on asymmetric molecules which were found to be down-regulated during blast crisis as compared to chronic phase. The regulation concordance between the two distinct cohorts was principally observed on asymmetric molecules down regulated during blast crisis as compared to chronic phase suggesting less of asymmetric properties during advanced phase of the disease. The down regulation in patients in blast crisis was confirmed at single cell level for the expression of SELL, CD63, NUMB, HK2 and LAMP2. CD63 expression has also been found to be of interest in CML chronic phase. Indeed, single cell neural network and deep learning in CP samples showed that the CD63 expression was the best asymmetric gene marker predicting a poor prognosis and this appeared to be induced by opposite regulation of HK2 and NUMB. In patients in CP-CML, the single cell trajectory reconstitution revealed the major regulatory role of CD63 highlighting a trajectory cluster implicating HSPB1, PIM2, ANXA5, LAMTOR1, CFL1, CD52, RAD52, MEIS1, PDIA3, all genes known to be involved also in hematopoietic malignancies such as myelodysplasia, AML and ALL.
Conclusion
We show here for the first time the identification of the expression of genes involved in the asymmetric division of CML HSC. Among these genes we identified the tetraspanin CD63 as being down-regulated during CML progression. The expression of the genes involved in the asymmetric HSC division should require further studies in CML, either to predict persistence of CML or progressive disease.
Figure 1 : Single cell transcriptome of Lin- CD34+ CD38- CML progenitors in CP links CD63 expression to progressive disease. A/ Piechart of counts for chronic phase cells analyzed according to prognosis; B/ pseudotime transformation of single cell transcriptome according patient prognosis; C/ Pseudotime expression heatmap; D/ Pseudotime expression of CD63 in CP.
Disclosures
No relevant conflicts of interest to declare.